 Our company has
grown in the past year, with two people added to our technical staff.
We are pleased to announce the additions of Steve Strack, Materials/Metallurgical
and Corrosion Engineer, and Doug Tannenbaum, Metallographer/Metallurgical
Technician. Our company has
grown in the past year, with two people added to our technical staff.
We are pleased to announce the additions of Steve Strack, Materials/Metallurgical
and Corrosion Engineer, and Doug Tannenbaum, Metallographer/Metallurgical
Technician.
Steve Strack (left) and Doug Tannenbaum
(right).
  
 Steve Strack
comes to us from Packer Engineering in Naperville, Illinois, where
he has spent the past eight years as a metallurgical/ corrosion
expert. His tenure at Packer included analysis of corrosion related
failures in the chemical processing and petroleum industries. Much
of his experience was in processing and manufacturing plants inspecting
failed equipment and diagnosing problems. Many of these corrosion
related problems involved heat exchangers, boilers, pumps, piping
components and process vessels, covering materials from carbon steel
to stainless steels, copper alloys, nickel alloys, aluminum alloys
and high temperature alloys. Failures also included non-metallics
such as fiberglass, PVC, paints and coatings. Steve not only did
work on corrosion problems, he also conducted laboratory failure
analysis of a variety of materials and components failing due to
fatigue, overload, material defects, improper manufacturing techniques
or operating parameters, solving problems and recommending corrective
actions. Steve Strack
comes to us from Packer Engineering in Naperville, Illinois, where
he has spent the past eight years as a metallurgical/ corrosion
expert. His tenure at Packer included analysis of corrosion related
failures in the chemical processing and petroleum industries. Much
of his experience was in processing and manufacturing plants inspecting
failed equipment and diagnosing problems. Many of these corrosion
related problems involved heat exchangers, boilers, pumps, piping
components and process vessels, covering materials from carbon steel
to stainless steels, copper alloys, nickel alloys, aluminum alloys
and high temperature alloys. Failures also included non-metallics
such as fiberglass, PVC, paints and coatings. Steve not only did
work on corrosion problems, he also conducted laboratory failure
analysis of a variety of materials and components failing due to
fatigue, overload, material defects, improper manufacturing techniques
or operating parameters, solving problems and recommending corrective
actions.
 Prior to joining
Packer, Steve spent 15 years solving materials problems as Materials/Corrosion
Manager with Velsicol Chemical Corporation, a supplier of pesticides,
herbicides, chlorinated organic compounds, brominated compounds,
organic/inorganic acids and plasticizers. He supported six operating
plants, corporate engineering, R&D and other corporate groups
on corrosion mitigation, failure analysis, material selection, equipment
inspection and corrosion testing. Prior to joining
Packer, Steve spent 15 years solving materials problems as Materials/Corrosion
Manager with Velsicol Chemical Corporation, a supplier of pesticides,
herbicides, chlorinated organic compounds, brominated compounds,
organic/inorganic acids and plasticizers. He supported six operating
plants, corporate engineering, R&D and other corporate groups
on corrosion mitigation, failure analysis, material selection, equipment
inspection and corrosion testing.
 Steve also has
experience as a Materials Engineer for several engineering/construction
firms in the chemical processing and petroleum industries. Steve
has an associate degree in Metallurgical Technology, and a BS degree
in Metallurgical Engineering from Michigan Technological University. Steve also has
experience as a Materials Engineer for several engineering/construction
firms in the chemical processing and petroleum industries. Steve
has an associate degree in Metallurgical Technology, and a BS degree
in Metallurgical Engineering from Michigan Technological University.
 As you can see,
Steve has extensive experience which he brings to MEi, complementing
our expertise in failure analysis, and expanding our expertise and
capabilities in corrosion, processing equipment and non-metals. As you can see,
Steve has extensive experience which he brings to MEi, complementing
our expertise in failure analysis, and expanding our expertise and
capabilities in corrosion, processing equipment and non-metals.
 Doug Tannenbaum
joined MEi in 1998 from Bodycote Taussig in Skokie, Illinois, bringing
additional expertise in metallography to us. After receiving his
BS degree in Science from the College of St. Francis in Joliet,
Illinois, Doug has spent his entire professional career in metallurgical
laboratories. He began his career in the lab at Caterpillar, conducting
hardness and mechanical testing, sample preparation, metallography,
NDT and operating the spectrometer. While at Taussig, he conducted
sample preparation, metallography, microhardness, macro and micro
photography and testing of non-metallics. Doug's experience covers
virtually every aspect of materials analysis and testing, and expands
our expertise and know how in these areas. Doug Tannenbaum
joined MEi in 1998 from Bodycote Taussig in Skokie, Illinois, bringing
additional expertise in metallography to us. After receiving his
BS degree in Science from the College of St. Francis in Joliet,
Illinois, Doug has spent his entire professional career in metallurgical
laboratories. He began his career in the lab at Caterpillar, conducting
hardness and mechanical testing, sample preparation, metallography,
NDT and operating the spectrometer. While at Taussig, he conducted
sample preparation, metallography, microhardness, macro and micro
photography and testing of non-metallics. Doug's experience covers
virtually every aspect of materials analysis and testing, and expands
our expertise and know how in these areas.
 Doug is taking
care of all metallurgical technician duties, including microscopy,
sample preparation, photography, microhardness and hardness. Doug
will also be sharing scanning electron microscope (SEM) and energy
dispersive spectroscopy (EDS) duties with the rest of our staff. Doug is taking
care of all metallurgical technician duties, including microscopy,
sample preparation, photography, microhardness and hardness. Doug
will also be sharing scanning electron microscope (SEM) and energy
dispersive spectroscopy (EDS) duties with the rest of our staff.
 We are excited
to have Steve and Doug at Materials Engineering, Inc. They will
allow us to continue to offer highly professional engineering and
metallurgical services, while remaining responsive to your schedule
needs as our business continues to grow. We are excited
to have Steve and Doug at Materials Engineering, Inc. They will
allow us to continue to offer highly professional engineering and
metallurgical services, while remaining responsive to your schedule
needs as our business continues to grow.

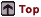
 Low carbon or
"L" grade stainless steels have been widely discussed
as offering improved corrosion resistance over regular grades. But
will using a L grade stainless offer improvements in your application,
or will you simply be wasting your money? By understanding their
metallurgy and history, you can answer this question. Low carbon or
"L" grade stainless steels have been widely discussed
as offering improved corrosion resistance over regular grades. But
will using a L grade stainless offer improvements in your application,
or will you simply be wasting your money? By understanding their
metallurgy and history, you can answer this question.
Background
 As early as
the 1930's it was well recognized that austenitic stainless steels
of the then termed"18-8" variety (referring to the 18%
chromium and 8% nickel composition of the first austenitic stainless
steel grades) occasionally suffered corrosion adjacent to but slightly
removed from welds when exposed to certain environments. The cause
was traced to the depletion of the effective chromium content within
that area resulting in a localized reduction in corrosion resistance. As early as
the 1930's it was well recognized that austenitic stainless steels
of the then termed"18-8" variety (referring to the 18%
chromium and 8% nickel composition of the first austenitic stainless
steel grades) occasionally suffered corrosion adjacent to but slightly
removed from welds when exposed to certain environments. The cause
was traced to the depletion of the effective chromium content within
that area resulting in a localized reduction in corrosion resistance.
Preferential corrosion in the HAZ of a weld
sample, indicated by arrows.
This condition is termed sensitization, and occurs in a region
one-sixteenth to one-quarter inch away from the weld known as the
heat affected zone, or HAZ. The corrosion is intergranular in nature,
attacking along the grain boundaries between the grains of the metal.
The chemical species which cause this type of corrosion are called
intergranular corrodents. When regular grade stainless steels are
exposed to temperatures between 900°F and 1400°F in the
HAZ during welding, the chromium combines with available carbon
forming chromium carbides (Cr23C6), which are useless in providing
corrosion resistance. The chromium carbides migrate to the grain
boundaries leaving a localized chromium depleted region in the grains
adjacent to the grain boundaries, resulting in lower corrosion resistance
than the rest of the material. Thus when exposed to certain environments
in which intergranular corrosion does occur in the HAZ, the corrosion
proceeds along the grain boundaries eventually causing individual
grains to fall out. Under the microscope the corroding surface has
the appearance of rock candy.
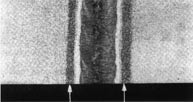
Metallographic cross section showing corrosion
at the grain boundaries in the HAZ. 
 125X 125X
 Closer to and
in the weld, the temperature is above the critical 900° to 1400°F
range, and any chromium carbides that form are re-dissolved into
the alloy. Farther away from the weld at temperatures below this
range, the carbide forming reaction is too sluggish to occur in
the short period of time during welding.
Metallurgical Improvements 
 Sensitization
can occur if the carbon content of the stainless steel is above
0.03%. Prior to World War II, steel making technology could only
produce stainless steels with carbon contents ranging from 0.05
to 0.08%. The first approach to alleviate sensitization was to add
what are called stabilizing elements, which are more reactive with
carbon than chromium. Small additions of titanium or columbium react
with the excess carbon forming carbides (TiC or CbC) before the
chromium does. 321 and 347 stainless steels are the resultant stabilized
grades that are still available today. However, they have their
own particular idiosyncrasies and are not always the best solution. Sensitization
can occur if the carbon content of the stainless steel is above
0.03%. Prior to World War II, steel making technology could only
produce stainless steels with carbon contents ranging from 0.05
to 0.08%. The first approach to alleviate sensitization was to add
what are called stabilizing elements, which are more reactive with
carbon than chromium. Small additions of titanium or columbium react
with the excess carbon forming carbides (TiC or CbC) before the
chromium does. 321 and 347 stainless steels are the resultant stabilized
grades that are still available today. However, they have their
own particular idiosyncrasies and are not always the best solution.
 With the advent
of steel making technology that permits commercial products with
carbon contents lower than 0.03%, the low carbon or "L"
grades were developed. These grades are virtually immune to sensitization.
The most common "L" grades are 304L and 316L, which are
identical to 304 and 316 except they contain less carbon. If additional
corrosion resistance is required, a higher chromium/molybdenum content
317L is also available. With the advent
of steel making technology that permits commercial products with
carbon contents lower than 0.03%, the low carbon or "L"
grades were developed. These grades are virtually immune to sensitization.
The most common "L" grades are 304L and 316L, which are
identical to 304 and 316 except they contain less carbon. If additional
corrosion resistance is required, a higher chromium/molybdenum content
317L is also available.
 Beyond alloy
selection, sensitization can be eliminated by solution annealing
the regular grades after welding. The exact recipe varies with the
grade of stainless steel, but generally involves heating to 1850
to 2050 F to dissolve all chromium carbides present. The material
must then be rapidly cooled to prevent chromium carbide reformation
as it passes through the critical 900 to 1400 F temperature range.
This, of course, cannot be applied to large components, and some
strength reduction and distortion must be taken into account. Beyond alloy
selection, sensitization can be eliminated by solution annealing
the regular grades after welding. The exact recipe varies with the
grade of stainless steel, but generally involves heating to 1850
to 2050 F to dissolve all chromium carbides present. The material
must then be rapidly cooled to prevent chromium carbide reformation
as it passes through the critical 900 to 1400 F temperature range.
This, of course, cannot be applied to large components, and some
strength reduction and distortion must be taken into account.
Should You Use "L" Grade Stainless
Steel? 
 The "L"
grades have been embraced with enthusiasm throughout industry to
the point that they have developed a reputation of being more corrosion
resistant than the regular grades, and usually command a premium
in pricing. In reality, they are only more corrosion resistant if
the material is sensitized and used in certain environments. The
environments that cause intergranular corrosion are not as widespread
as may be imagined. The "L"
grades have been embraced with enthusiasm throughout industry to
the point that they have developed a reputation of being more corrosion
resistant than the regular grades, and usually command a premium
in pricing. In reality, they are only more corrosion resistant if
the material is sensitized and used in certain environments. The
environments that cause intergranular corrosion are not as widespread
as may be imagined.
SEM photograph showing intergranular corrosion
and a "rock candy" surface appearance in the HAZ.

 125X 125X
In general, they consist of acidic environments that are not corrosive
to stainless steel containing 18% effective chromium, but are corrosive
to stainless steel containing only 8-12% effective chromium. If
your parts are not destined for such service, using an "L"
grade may be an expensive luxury.
 As a final note,
since there is no lower limit on carbon, some items such as piping
are being marketed as complying to both "L" grade and
regular grade material. This may be detrimental since the "L"
grades have 5-10% lower yield strength up to 1000 F, and are not
recommended for service above 1200 F. If strength or fatigue resistance
are critical for your components, it would be wise to determine
the actual carbon content of your raw material. As a final note,
since there is no lower limit on carbon, some items such as piping
are being marketed as complying to both "L" grade and
regular grade material. This may be detrimental since the "L"
grades have 5-10% lower yield strength up to 1000 F, and are not
recommended for service above 1200 F. If strength or fatigue resistance
are critical for your components, it would be wise to determine
the actual carbon content of your raw material.
Summary 
 The "L"
grades are an important part of the stainless steel arsenal in the
fight against corrosion, primarily for specific corrosion problems
involving the production and use of chemicals. If you are unsure
if your parts need to be "L" grade or not, give us a call
as we would be pleased to discuss it with you. The "L"
grades are an important part of the stainless steel arsenal in the
fight against corrosion, primarily for specific corrosion problems
involving the production and use of chemicals. If you are unsure
if your parts need to be "L" grade or not, give us a call
as we would be pleased to discuss it with you.

 Over the past
year, you may have noticed a customer satisfaction survey in with
your report. The survey allows you to grade us (from A to F) in
such areas as technical competence, courtesy of staff, communications,
cost, report quality and timeliness of results, as well as provides
an opportunity to make general comments and complaints. We know
you, our customer, the best source of information on how well we
are meeting your needs. Over the past
year, you may have noticed a customer satisfaction survey in with
your report. The survey allows you to grade us (from A to F) in
such areas as technical competence, courtesy of staff, communications,
cost, report quality and timeliness of results, as well as provides
an opportunity to make general comments and complaints. We know
you, our customer, the best source of information on how well we
are meeting your needs.
 We have recently
reviewed your responses, and are happy with the high level of satisfaction
you have shown. While we did not quite achieve a "4.0"
grade point average, we would have made most honor rolls. We have recently
reviewed your responses, and are happy with the high level of satisfaction
you have shown. While we did not quite achieve a "4.0"
grade point average, we would have made most honor rolls.
Some of the comments received were:
 "Your service
is Great. It is nice to have a good external engineer to consult
with." "Your service
is Great. It is nice to have a good external engineer to consult
with."
 "At last,
a lab I can communicate with." "At last,
a lab I can communicate with."
 "The size
of your company affords you the luxury of staying focused on the
things "The size
of your company affords you the luxury of staying focused on the
things
that are important. I am very satisfied with the work done."
 "As usual,
MEi provides us with the right amount of information and detail
for what "As usual,
MEi provides us with the right amount of information and detail
for what
we need to accomplish."
 "Very nice
job, my customer was very happy with the professional results." "Very nice
job, my customer was very happy with the professional results."
 "We consider
MEi our "in house" metallurgist." "We consider
MEi our "in house" metallurgist."
 Our highest marks
were in timelines of results (81% A's) and courtesy of staff (91%
A's) All of the respondents indicated they would use MEi again,
and would recommend MEi to others. Our highest marks
were in timelines of results (81% A's) and courtesy of staff (91%
A's) All of the respondents indicated they would use MEi again,
and would recommend MEi to others.
 Thank you for
your input. We plan to do everything possible to continue to keep
you satisfied in the future, and we look forward to hearing your
comments. Thank you for
your input. We plan to do everything possible to continue to keep
you satisfied in the future, and we look forward to hearing your
comments.

 The nature of
our business puts us in contact with many people working on improving
their products. They are often looking for sources of materials,
processing, products, coatings and technologies. We are often asked
"Do you know of anyone who...?" We keep extensive technical
files and can often help to point someone to a good source, but
not always. We are hoping you can help us. The nature of
our business puts us in contact with many people working on improving
their products. They are often looking for sources of materials,
processing, products, coatings and technologies. We are often asked
"Do you know of anyone who...?" We keep extensive technical
files and can often help to point someone to a good source, but
not always. We are hoping you can help us.
 We have a general
understanding of the products and service available from many of
our customers, but our knowledge in not complete. If we had more
knowledge on the complete extent of the products and services that
you provide, we may be able to point potential customers toward
your products or services to assist them. We have a general
understanding of the products and service available from many of
our customers, but our knowledge in not complete. If we had more
knowledge on the complete extent of the products and services that
you provide, we may be able to point potential customers toward
your products or services to assist them.
 If you can,
we are requesting you to send to us information describing the range
of products that your company offers, or the services you provide.
This does not apply just to materials suppliers or materials processors,
but to all customers. You might be surprised at the range of products
which are of interest to our other customers. So mail your marketing
literature to Bill's attention at our address. Thanks. If you can,
we are requesting you to send to us information describing the range
of products that your company offers, or the services you provide.
This does not apply just to materials suppliers or materials processors,
but to all customers. You might be surprised at the range of products
which are of interest to our other customers. So mail your marketing
literature to Bill's attention at our address. Thanks.

 The scanning
electron microscope (SEM) is a powerful tool, capable of magnifications
up to 180,000 times. It allows us to reveal information which is
critical to metallurgical investigations, such as fracture modes
and surface characteristics. The scanning
electron microscope (SEM) is a powerful tool, capable of magnifications
up to 180,000 times. It allows us to reveal information which is
critical to metallurgical investigations, such as fracture modes
and surface characteristics.
 The SEM can also
be fun to play with, because it allows one to view the surface of
anything at high magnification with great depth of field. All of
us have been amazed by the pictures of various insect parts, especially
the eye of a fly. The SEM can also
be fun to play with, because it allows one to view the surface of
anything at high magnification with great depth of field. All of
us have been amazed by the pictures of various insect parts, especially
the eye of a fly.
 In our contest,
we take a look at an object on the SEM that should be familiar to
all of you.In this issue, our photographs show a natural substance
from our 50th state, at two different magnifications. Good Luck. In our contest,
we take a look at an object on the SEM that should be familiar to
all of you.In this issue, our photographs show a natural substance
from our 50th state, at two different magnifications. Good Luck.
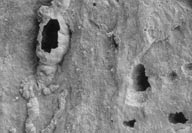  
 60X 60X 900X
900X  Please
fax, mail or e-mail us (don't call) with your answer. We will draw
a winner from all correct entries received by June 6. The correct
answer and the winner will be published in the next issue Of Materials
Interest. Please
fax, mail or e-mail us (don't call) with your answer. We will draw
a winner from all correct entries received by June 6. The correct
answer and the winner will be published in the next issue Of Materials
Interest.
 The prize is
a $50 restaurant gift certificate, so put on your thinking caps. The prize is
a $50 restaurant gift certificate, so put on your thinking caps.
Results:
 Last issue, we
provided the clue that 'might be found in your kitchen, but you
really wouldn't want to eat it.' While our favorite answer came
from Andy Johnson at Excel of Iowa, who knew just from the clue
that it had to be quiche, the correct answer was mold. Last issue, we
provided the clue that 'might be found in your kitchen, but you
really wouldn't want to eat it.' While our favorite answer came
from Andy Johnson at Excel of Iowa, who knew just from the clue
that it had to be quiche, the correct answer was mold.
 Our winner,
drawn at random from the correct entries, was Ed Gade of Taylor
Company in Rockton, Illinois. His efforts earned he and his wife
a nice dinner at Cliffbreakers Restaurant in Rockford. Taylor Company
designs and manufactures food service equipment, including soft
serve ice cream and frozen yogurt machines, shake machine, and cooking
grills for fast food and restaurants, such as McDonalds. Look for
the Taylor name on the machine next time you order an ice cream
cone. You can learn more about them at www.taylor-company.com Our winner,
drawn at random from the correct entries, was Ed Gade of Taylor
Company in Rockton, Illinois. His efforts earned he and his wife
a nice dinner at Cliffbreakers Restaurant in Rockford. Taylor Company
designs and manufactures food service equipment, including soft
serve ice cream and frozen yogurt machines, shake machine, and cooking
grills for fast food and restaurants, such as McDonalds. Look for
the Taylor name on the machine next time you order an ice cream
cone. You can learn more about them at www.taylor-company.com
 Congratulations,
Ed. Congratulations,
Ed. |